The Covid-19 Vaccine Shell Game, the Defense Department, and the Damage to Our Military and National Security
The Damage Done
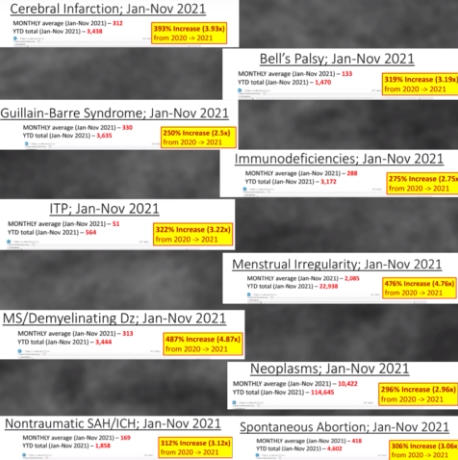
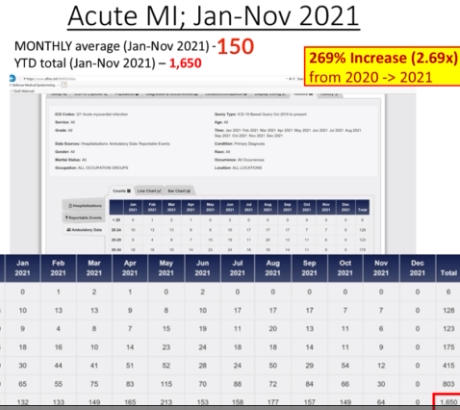
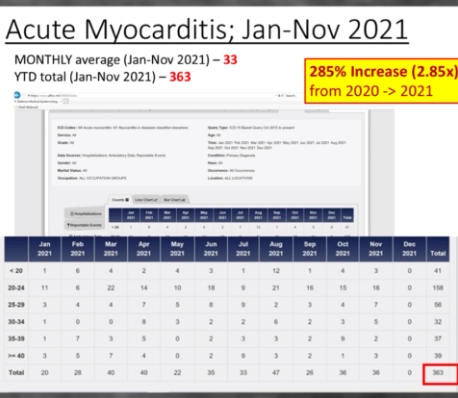
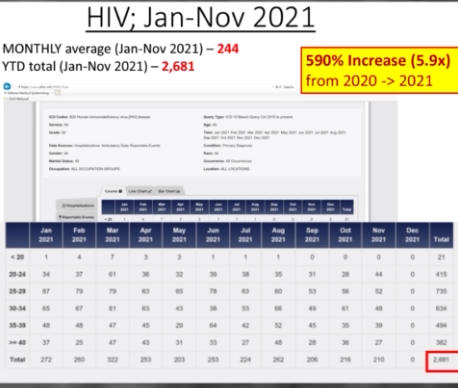
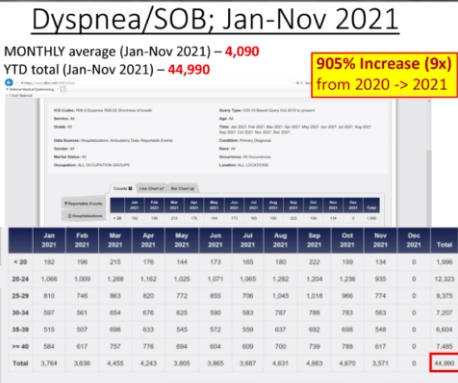
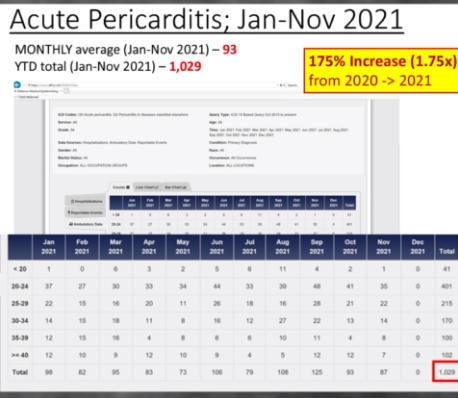
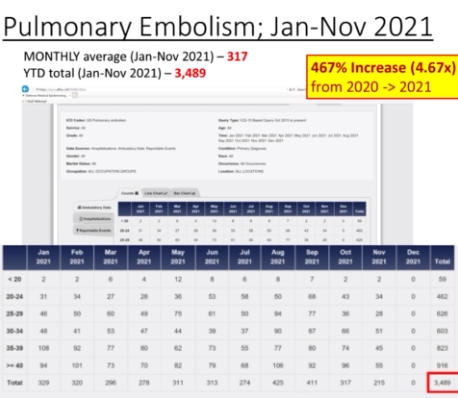
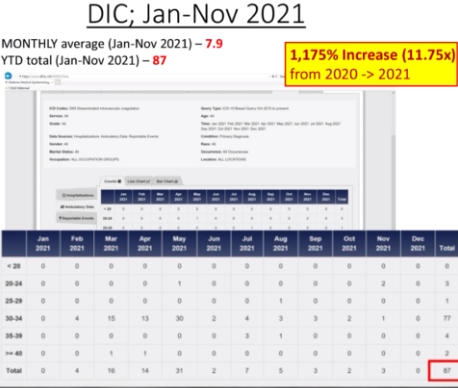
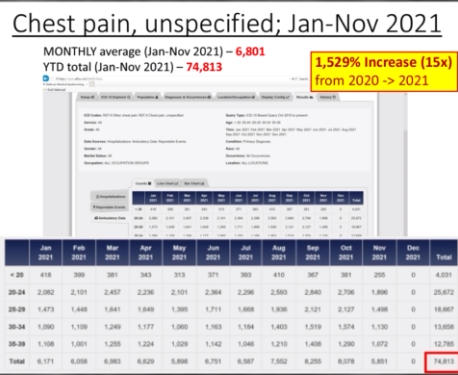
The Department of Defense’s own counterpart to VAERS is DMED, and a whistleblower released documents showing the widespread damage that the mandatory Covid-19 vaccine program has had on our military servicemen and women. The numbers of increased illnesses are truly shocking and sickening. In the past year, the number of patients recorded by the DoD’s own data shows increases in heart attacks, immune system deficiencies, neurological disorders, and blood diseases in the several 100’s of percent. Sadly, the higher ups lied about the Pfizer Covid-19 vaccine status in order to make them mandatory to all servicemen in the Armed Forces. The video below by Terminal CWO explains the cover-up and the corruption by the Biden Administrations’ own Secretary of Defense Lloyd Austin III, and his huge profiting from, no doubt, a government contract with Pfizer for their Covid-19 vaccines.
From the Blaze:
According to the military, DMED is the Armed Forces Health Surveillance Branch’s (AFHSB) “web-based tool to remotely query de-identified active component personnel and medical event data contained within the Defense Medical Surveillance System (DMSS).” In other words, it contains every ICD medical billing code for any medical diagnosis in the military submitted for medical insurance billing during any given period of time. Three military doctors have presented queried data to Renz that shows a shocking and sudden spike in nearly every ICD code for common vaccine injuries in 2021.
In a declaration under penalty of perjury that Renz plans to use in federal court, Dr. Samuel Sigoloff, Peter Chambers, and Theresa Long — three military doctors — revealed that there has been a 300% increase in DMED codes registered for miscarriages in the military in 2021 over the five-year average. The five-year average was 1,499 codes for miscarriages per year. During the first 10 months of 2021, it was 4,182.
Attorney Tom Renz speaking at the Covid-19: A Second Opinion Conference
The Law Ignored
It appears that upon the advise of Dawn Johnsen, Acting Assistant Attorney General in the Office of Legal Counsel for the Biden Administration she tried to give the Secretary of Defense Lloyd Austin legal cover to bypass the FDA’s existing regulations in order to push through the Covid-19 vaccine mandate upon our U.S. military. The follow are comments from her Memorandum Opinion for the Deputy Counsel to the President:
As noted above, FDA agrees with our interpretation of section 564. On a few occasions, however, FDA has made statements that could be understood as saying that the condition described in section 564(e)(1)(A)(ii)(III) prohibits entities (particularly the U.S. military) from requiring the use of EUA products. In 2005, for instance, FDA issued an EUA that permitted the use of a vaccine for the prevention of inhalation anthrax by individuals between 18 and 65 years of age who were deemed by the Department of Defense (“DOD”) to be at heightened risk of exposure due to an attack with anthrax. As a condition of that authorization, the agency required DOD to inform potential vaccine recipients “of the option to accept or refuse administration of [the vaccine].” Authorization of Emergency Use of Anthrax Vaccine Adsorbed for Prevention of Inhalation Anthrax by Individuals at Heightened Risk of Exposure Due to Attack With Anthrax; Availability, 70 Fed. Reg. 5452, 5455 (Feb. 2, 2005).
That EUA continued: With respect to [the] condition . . . relating to the option to accept or refuse administration of [the vaccine], the [immunization program] will be revised to give personnel the option to refuse vaccination. Individuals who refuse anthrax vaccination will not be punished. Refusal may not be grounds for any disciplinary action under the Uniform Code of Military Justice. Refusal may not be grounds for any adverse personnel action. Nor would either military or civilian personnel be considered non-deployable or processed for separation based on refusal of anthrax vaccination. There may be no penalty or loss of entitlement for refusing anthrax vaccination.
As for DOD’s concern about service members who would lack a meaningful option to refuse EUA products because of the prospect of sanction, including possibly prosecution, we note that any difference between our view and the assumption reflected in the conference report should have limited practical significance. Given that FDA has imposed the “option to accept or refuse” condition for the COVID-19 vaccines by requiring distribution of its Fact Sheet containing the “[i]t is your choice to receive or not receive” language, DOD is required to provide service members with the specified notification unless the President waives the condition pursuant to 10 U.S.C. § 1107a. And because DOD has informed us that it understandably does not want to convey inaccurate or confusing information to service members—that is, telling them that they have the “option” to refuse the COVID-19 vaccine if they effectively lack such an
option because of a military order—DOD should seek a presidential waiver before it imposes a vaccination requirement. III.For the reasons set forth above, we conclude that section 564 of the FDCA does not prohibit public or private entities from imposing vaccination requirements, even when the only vaccines available are those authorized under EUAs.
DAWN JOHNSEN
Acting Assistant Attorney General
Office of Legal Counsel
What is very telling about this is that at a time when the U.S. was suffering from domestic and foreign terrorism through the form of Anthrax attacks, the FDA still allowed U.S. military members the option to refuse the vaccines without the penalty of non-deployments, Article 15’s or Dishonorable Discharges. This current leadership in the Defense Dept. or the Biden Administration has not been so generous with individual freedoms, despite Covid-19 being of little consequence to healthy and fit people, which includes a majority of those serving in the Armed Forces.
The Vaccine Shell Game
Secretary of Defense Lloyd Austin issued a mandate for servicemen to get the Covid-19 vaccines, namely, the Pfizer-BioTech (Emergency Use Authorization) vaccine as approved by the FDA. Seven weeks after this mandate, 16 servicemen sued the Secretary and others challenging the mandate. There are (2) versions of the Pfizer-BioTech Covid-19 vaccines; (1) Pfizer-BioNTech EUA (Emergency Use Authorization) version and (2) the Pfizer-BioNTech BLA (fully FDA approved for use) version registered under the product label “Comirnaty.”
PandemicTimeline.com makes (4) very good distinctions between the (2) vaccines:
- The Pfizer-BioNTech EUA jab comes in packaging that states that informed consent is necessary for administration due to its experimental nature. The packaging of the Comirnaty jab does not have a statement about informed consent or being experimental. This is a legal distinction between the two products.
- The approved Comirnaty jab may only be manufactured in BLA-approved manufacturing facilities. There is no indication that all EUA-labeled vials are from BLA-approved facilities. If the vial of jab was manufactured in a non-BLA-approved facility, then it can in no way be considered approved, no matter what the formulation is.
- Only certain lots of the Pfizer-BioNTech EUA jabs are of the same formulation as the approved Comirnaty jab. It is necessary to know the lot number of a Pfizer-BioNTech EUA jab to know its formulation.
- For those in the military, please note that this court ruling states that “The DOD acknowledges that the President has not executed a wavier under this section, ECF No. 45 at 52:8-9, so as things now stand, the DOD cannot mandate vaccines that only have an EUA.” In other words, only approved jabs can be mandated. “[T]he DOD concedes that some of its current vials are not BLA-compliant, and that there is no policy to ensure that servicemembers get only BLA-compliant vaccines.” The EUA jabs can only be used with a presidential waiver. And since no presidential waiver was executed, anyone who has given you an EUA jab against your will or under duress has committed an unlawful act. The order is lawfully fulfilled only when approved jabs are administered.
The Department of Defense made the claim that the (2) vaccines were simply interchangeable, and U.S. Federal District Judge Allen Winsor of the U.S. District Court for the Northern District of Florida denied the DoD’s claim. You can read his ruling in Military Mandate TRO Denial Florida (Doe v. Austin) on (Page 10):
Under the EUA statute, recipients of EUA drugs must be “informed . . . of the option to accept or refuse administration of the product.” 21 U.S.C. § 360bbb-3(e)(1)(A)(ii)(III); see also 5 U.S.C. § 706(2)(C) (APA provision prohibiting agency action taken “in excess of statutory jurisdiction, authority, or limitations, or short of statutory right”). And under 10 U.S.C. § 1107a, “[i]n the case of the administration of [an EUA] product . . . to members of the armed forces,” that statutory right to refuse “may be waived only by the President only if the President determines, in writing, that complying with such requirement is not in the interests of national security.” 10 U.S.C. § 1107a(a)(1). The DOD acknowledges that the President has not executed a wavier under this section, ECF No. 45 at 52:8-9, so as things now stand, the DOD cannot mandate vaccines that only have an EUA. 10 U.S.C. §
The DOD’s interpretation of § 1107a is unconvincing. For starters, FDA licensure does not retroactively apply to vials shipped before BLA approval. See 21 U.S.C. § 355(a) (“No person shall introduce . . . into interstate commerce any new drug, unless an approval of an application [for FDA licensure] is effective with respect to such drug.” (emphasis added)). Thus, as a legal matter, vaccines sent before August 23—and vaccines produced after August 23 in unapproved facilities—remain “product[s] authorized for emergency use under section 564 of the Federal Food, Drug, and Cosmetic Act.” § 1107a(a)(1).9 Section 1107a’s explicit cross-reference to the EUA provisions suggests a concern that drugs mandated for military personnel be actually BLA-approved, not merely chemically similar to a BLA-approved drug. And the distinction is more than mere labeling: to be BLA compliant, the drug must be produced at approved facilities, see ECF No. 1-4 at 2; 21 C.F.R. §§ 600.11, 600.20-.21, and there is no indication that all EUA-labeled vials are from BLA-approved facilities.10 Moreover, the DOD concedes that some of its current vials are not BLA-compliant, and that there is no policy to ensure that servicemembers get only BLA-compliant vaccines. See ECF No. 45 at 61:10-12. It is difficult to see how vials that the DOD admits are not BLA-compliant—and thus could only be EUA products—could fall outside § 1107a’s prohibition on mandatory administration.
First, it is worth noting that the (2) Covid-19 vaccines were ruled as NOT interchangeable. Second, the DoD cannot mandate Covid-19 vaccines that are only EUA status from the FDA, unless the President executes a wavier for this product. Currently, it does not appear that President Biden has provided such a wavier for the Covid-19 EUA vaccines to be mandatory in the U.S. military, however, the military leadership could be mandating it within their own ranks regardless.
It has been reported that the FDA has approved the Pfizer-BioNTech Covid-19 vaccine product known as “Comirnaty” to the BLA status, allowing it full usage. However, Pfizer-BioNTech has no interest in actually producing this new BLA licensed Covid-19 vaccine, according to Daily Med on the NIH.gov website:
Pfizer received FDA BLA license on 8/23/2021 for its COVID-19 vaccine for use in individuals 16 and older (COMIRNATY). At that time, the FDA published a BLA package insert that included the approved new COVID-19 vaccine tradename COMIRNATY and listed 2 new NDCs (0069-1000-03, 0069-1000-02) and images of labels with the new tradename.
At present, Pfizer does not plan to produce any product with these new NDCs and labels over the next few months while EUA authorized product is still available and being made available for U.S. distribution. As such, the CDC, AMA, and drug compendia may not publish these new codes until Pfizer has determined when the product will be produced with the BLA labels.
For your safety the product labeling looks like this:
However, there is some suspicion as to the FDA’s BLA status for Pfizer-BioNTech’s Covid-19 vaccine, as at one point the expiration date was on the same day it was approved, but was later removed from the website.
Another point which may garner some further investigation is the link between the almost 30,000 shares of Tenet Healthcare Corp. which would benefit largely from a Covid-19 vaccine mandate. It is worth noting that upon Sec. Def. Lloyd Austin taking his new position in the Biden Admin., he had 120 days to unload his shares from his previous employer of Tenet Healthcare Corp.. Any personal arrangement like a golden parachute or bonus may not be a matter of public record, but would deserve further investigation on how exactly he may have personally profited from a Tenet Healthcare/Covid-19 vaccine mandate in the military.
There is an elaborate Pfizer-BioNTech Covid-19 vaccine shell game going on between the EUA version of the Pfizer-BioNTech and the FDA approved BLA version of the Pfizer-BioNTech Covid-19 vaccine [U.S. License No. 2229]. Sadly, the real victims in this shell game are our U.S. military servicemen and women, stuck between choosing between their individual health and mobility and their military careers in defending our great nation. These are not the kind of decisions our loyal soldiers should have to face. Clearly, more needs to be investigated in this vaccine campaign geared toward pushing profits over patients. I encourage you all to contact your Congressional members to push for investigations in the Covid-19 vaccine mandates damaging the lives of countless military families and our national security.













Leave a Reply